- Systematic reviews/meta-analyses (SRs/MAs) of moderate to high quality studies with consistent findings
- High quality randomized, controlled trials (RCTs)
- A high quality RCT reports at least 4 of the following:
- Allocation concealment
- Blinding, if possible
- Intention-to-treat analysis
- Adequate statistical power
- > 80% follow-up
Methodology
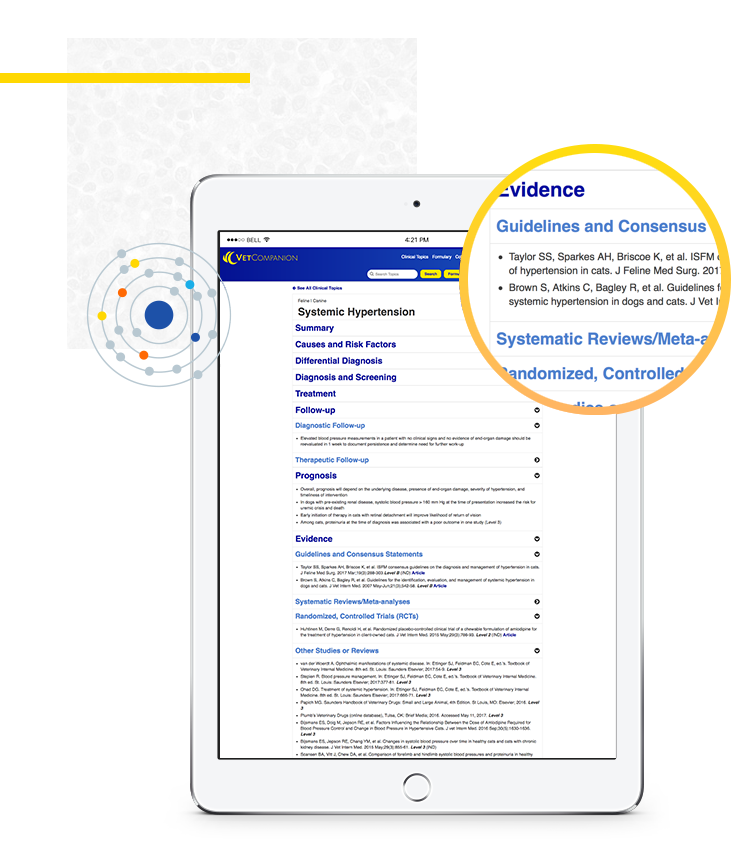
The evidence-based clinical content in VetCompanion has been developed by veterinary and evidence-based medicine experts using the latest guidelines, consensus statements, systematic reviews, randomized controlled trials, and other pertinent studies.
What is Evidence-Based Veterinary Medicine?
Evidence-based medicine (EBM) was described in 1996 as the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence-based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research (Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71–2). EBM has become widely accepted in human medicine and serves as the foundation for point-of-care clinical decision support resources for physicians.
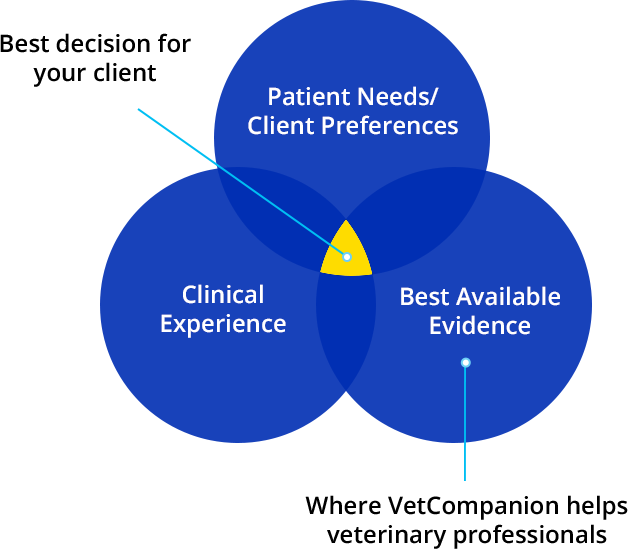
Evidence-based veterinary medicine (EBVM) involves the incorporation of EBM principles into the veterinary profession. It has been described as a formal strategy to integrate the best research evidence available, combined with clinical expertise as well as the unique needs or wishes of each client in clinical practice; much being based on results from research studies that have been critically-designed and statistically evaluated (Evidence-Based Veterinary Medicine Association [EBVMA], ebvma.org). EBVM is not “cookbook” veterinary practice. The evidence should inform the clinician, who will then use their expertise to determine if and how the available evidence applies to an individual patient, and how that evidence should influence clinical decisions.
EBVM has lagged behind EBM as many veterinarians do not have experience with or a clear understanding of how EBVM may impact the way they practice. High-quality primary research is somewhat lacking in veterinary medicine. Where there is research evidence, there are few secondary resources, similar to the Cochrane Collaboration in human medicine, which critically appraise and summarize the veterinary literature. According to an article on the EBVMA website, “The information technology infrastructure for making evidence available when and where it is needed by clinicians making decisions about individual patients is seriously underdeveloped in the veterinary field.” (McKenzie B. The future of evidence-based veterinary medicine: challenges and opportunities. 2013. ebvma.org)
Monograph Development
VetCompanion topics are chosen based on ongoing consultation with practicing veterinarians and members of the VetCompanion editorial board, review of relevant new guidelines, consensus statements, and new research studies that become available, review of frequently asked questions posted on veterinary consultation websites, and suggestions from VetCompanion users.
These monographs represent the most pertinent and commonly encountered conditions in veterinary medicine, as well as topics of significant clinical utility, such as fluid therapy and transfusion therapy. Topics are being added continuously, to provide an ever-growing resource for busy veterinary professionals. The goal is to provide the most comprehensive and trusted point-of-care resource available for veterinary professionals who care for small animals.
VetCompanion monographs are developed using a variety of resources to ensure that the existing evidence for any specific topic is thoroughly reviewed and accurately presented. The following resources are reviewed for all monographs, as appropriate:

VetMed Resource
CAB International

U.S. National Library of Medicine, National Institutes of Health
U.S. National Library of Medicine, National Institutes of Health

Database of veterinary systematic reviews
The University of Nottingham Centre for Evidence-Based Veterinary Medicine

A review of the best evidence available
The University of Nottingham Centre for Evidence-Based Veterinary Medicine

A Diagnostic Support System for Veterinary Medicine
Cornell University College of Veterinary Medicine

ACVIM Consensus Statements and Review Articles
American College of Veterinary Internal Medicine

AAHA Guidelines
American Animal Hospital Association

AAFP Practice Guidelines
American Association of Feline Practitioners

BSAVA Position Statements
British Small Animal Veterinary Association

Guidelines
International Society for Companion Animal Infectious Diseases

Evidence-Based Veterinary Medicine Association
- Other veterinary professional websites, as appropriate
- Textbooks – a minimum of 2 recent edition veterinary medicine textbooks are referenced for each monograph
Evidence Ratings
The available evidence for each clinical topic in VetCompanion is reviewed and pertinent citations are included in the evidence section of each monograph. An evidence rating is provided for each citation, to characterize for users the degree to which a given topic is supported by evidence. Links are provided for guidelines, consensus statements, and studies with higher evidence ratings (Level 1 or 2), so that users may more easily access and review the best supporting evidence at their convenience.
The VetCompanion evidence rating system has been designed conservatively, so citations will tend to be rated lower in the absence of evidence meeting specific, rigorous criteria. The evidence rating system is derived from The Strength-of-Recommendation Taxonomy (SORT), which is endorsed by the American Academy of Family Physicians. Some human medicine decision support, point-of-care resources use systems similar to or derived from SORT.
The following types of documents or studies are reviewed for each monograph, if available:
- Guidelines
- Consensus and position statements
- Systematic reviews
- Meta-analyses
- Randomized, controlled trials
- Observational studies, including cohort and case-control studies
- Other studies, such as cross-sectional studies and non-randomized experimental studies
- Case series and case reports
- Review articles, which may reflect expert opinion
For studies and review articles, the following evidence rating criteria are used:
LEVEL
1
LEVEL
2
- SRs/MAs not meeting or reporting Level 1 criteria
- RCTs not meeting or reporting Level 1 criteria
- Observational studies (cohort and case-control studies)
LEVEL
3
- Other studies not meeting criteria for Level 1 or 2, such as:
- Descriptive studies, including cross-sectional studies
- Non-randomized experimental studies
- Case series and case reports
- Review articles reflecting expert opinion and consensus
For guidelines and consensus/position statements, the following evidence rating criteria are used:
LEVEL
A
- Consistent, high quality research-based evidence to support recommendations
- Reports adequate number of good quality studies with consistent results
LEVEL
B
- Inconsistent, lower quality research-based evidence to support recommendations, OR
- Does not report adequate number of good quality studies with consistent results
LEVEL
C
- Research-based evidence not used to support recommendations
- Recommendations based solely on expert opinion or group/panel consensus
All pertinent guidelines, consensus statements, systematic reviews, meta-analyses, randomized, controlled trials, and observational studies are included for each clinical topic. The most recent and/or pertinent Level 3 studies and reviews are selected for inclusion in each monograph.
Editorial Policies
Currency of information
VetCompanion editorial staff update content on an ongoing basis, to ensure it reflects the most current evidence-based information available. This involves regular review of a variety of resources to identify new and pertinent evidence that may augment or change existing clinical content. When new clinical recommendations are published, such as new guidelines, the information is added or updated as soon as possible, as appropriate. Decisions regarding whether new evidence is sufficient to merit a revision of existing content are made by VetCompanion editorial staff.
Due to the rapidly changing nature of pharmacological information, users are encouraged to reference a reputable veterinary drug formulary as needed to confirm medication doses and obtain detailed information on adverse effects and contraindications.
Industry-sponsored studies and authors
VetCompanion will not cite or include references from sources that are sponsored by commercial enterprises/companies or written by authors who report sponsorship from commercial enterprises/companies, unless there are clearly stated conflict of interest policies in place and described that ensure any such studies, guidelines, or recommendations are not subject to potential biases due to such industry funding or sponsorship.
Absent that, VetCompanion will include studies, guidelines, and recommendations that are published in peer-reviewed journals or that explicitly state that they have been independently peer-reviewed. Such citations will be identified in the evidence section of monographs with the following designation: (IND). This designation will appear after the evidence rating (Level 1, 2, or 3). Such designation does not confirm that industry sponsorship-related bias exists, but indicates that readers should apply their own judgment regarding the potential for any such bias in the provided evidence.
Evidence ratings are based solely on the VetCompanion evidence rating methodology and do not take into account the potential for industry sponsorship-related bias. For more information on industry sponsorship and research outcomes, please review this link from the Cochrane Collaboration.
Conflict of interest
All members of the VetCompanion editorial board sign a conflict of interest form, declaring that they are not currently involved with any commercial interest in the veterinary field, in a compensated role, such as a paid member of an advisory panel. They also agree to immediately notify the VetCompanion editor-in-chief if they plan to take on such a role in the future and to voluntarily step down from the editorial board prior to beginning any such engagement. In signing the form, editorial board members confirm that they have no relevant conflicts of interest to report to the VetCompanion editorial staff.
Peer review
The Chief Veterinary Medical Officer and Editor-in-Chief review all VetCompanion content, including new monographs and updates. Members of the editorial board are responsible for peer reviewing selected VetCompanion monographs. In addition, a panel of veterinary specialists provides peer review of VetCompanion topics. Finally, content-related comments from VetCompanion users are reviewed, with revisions made as appropriate.